Sections D.5-D.7 Questions
By Kelly Moran
Questions 1-6 on pg. 131
Questions 2, 4, 6 on pg. 140
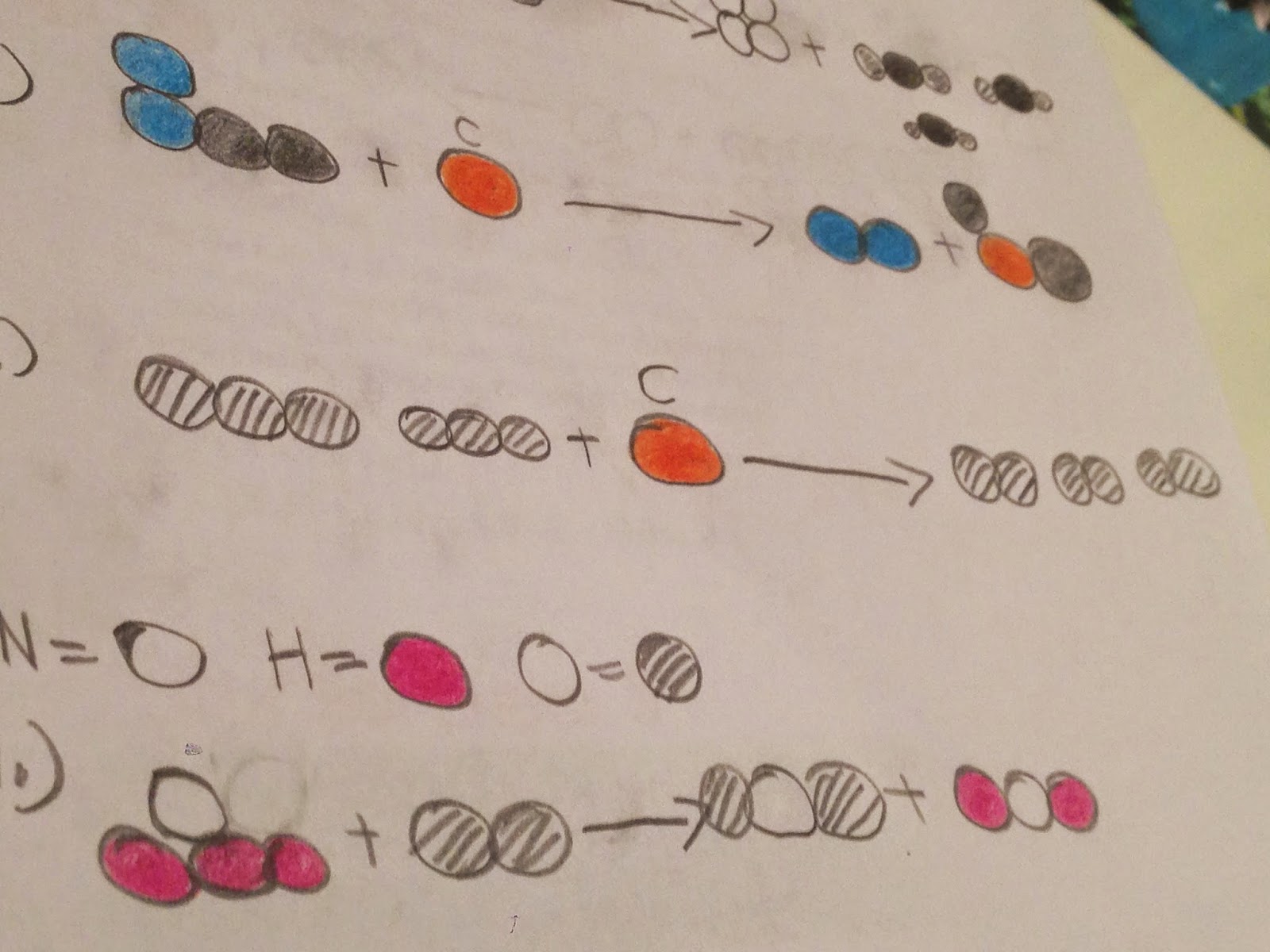
2.) a.) NaHCO2 + HCl = NaCl + H2O+ CO2
b.) C6H12O6 + O6 = 6CO2 + 6 H2O
4.) Scientific law is a statement based on repeated experimental observations and always implies that there is a casual relationship involving elements.
6.) "Using up" and "Throwing away" is incorrect and misleading when considering the law of conservation of matter because no atoms or thrown away they just get changed. Even when you burn wood, it may look like it has disappeared but it has really changed into smoke, gas, or ashes.





No comments:
Post a Comment