Global Cooling Science Report
By Kelly Moran
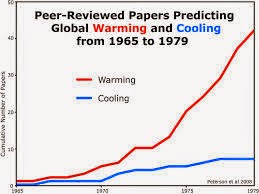
Global cooling was a popular conjecture during the 1970's that claimed that soon the world would go through another ice age. The popular myth had little scientific support but gained popularity through slight differing temperatures from the 1940's through the 1970's. The temperature had lowered through this period. Since 1945, temperatures started lowering on Earth even though many scientists believed in future warming of the earth due to Greenhouse gases. The general public during this time period were not aware that the release of carbon dioxide could cause the Earth to become warmer. The theory of Global cooling came during the 1970's but was immediately opposed by science magazines which claimed that Greenhouse gases would in fact increase global temperatures and right at this time the trend of downward temperatures changed to increasing temperatures. Currently though, there are many concerns about regional cooling due to the shutdown of thermohaline which is a side effect of global warming. This theory states that once global temperatures get high enough, fresh water will be coming into the North Atlantic and melt glaciers and because of this there will be a shutdown of thermohaline circulation. This could trigger localized cooling in the North Atlantic. Although this theory has been out for a bit, it has been proven to be untrue because even in models where the THC weakens global warming still continues. There is also another theory in which both global warming and global cooling are uncontrollable among humans. For instance like the ice age, there will be stages that the earth goes through from the sun and there is nothing humans can do about those stages. There are certain patterns that the earth goes through with temperatures therefore many scientists think that global cooling and warming are patterns that we cannot control. It is possible that we are going through a global warming stage right now that could possibly be the opposite of the ice age. The coming ice age was supposedly coming in the 70's but now it seems that a heat age is coming. Of course another theory (the most popular theory) with global warming is that greenhouse gases are released from automobiles, people, and other burnings of natural gases which all together create more carbon dioxide in the atmosphere which makes temperatures on the earth rise. Thank you!
Sources: http://en.wikipedia.org/wiki/Global_cooling
http://en.wikipedia.org/wiki/Shutdown_of_thermohaline_circulation
https://answers.yahoo.com/question/index?qid=20140722093941AAZls7K